©2025 Cognivue, Inc. | Privacy Policy | References
Lead the way in digital cognitive assessment
The Cognivue Clarity® device is a self-administered, digital cognitive assessment that standardizes the patient’s experience and removes the bias that can exist with traditional testing methods. Our proprietary technology collects over 130,000 data points and is highly sensitive to each individual patient. Using adaptive psychophysics, the test dynamically adapts as the patient moves through the test.
Self-Administered
Objective, self-administered test that removes the potential for bias
10 Minutes
10-minute test based on FDA‑cleared technology
Comprehensive Report
Automated reporting generates an easy-to-understand report
Dynamic Testing
Unique, customized, patient experience
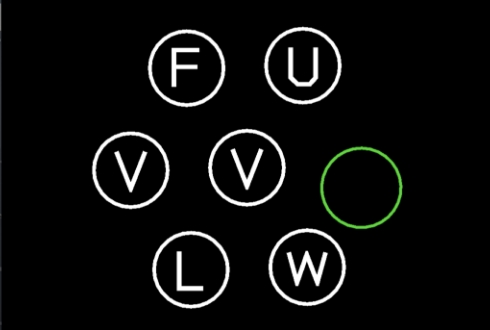
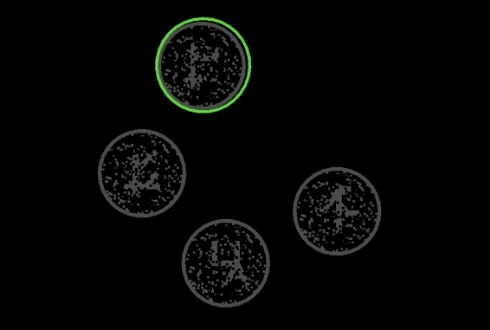
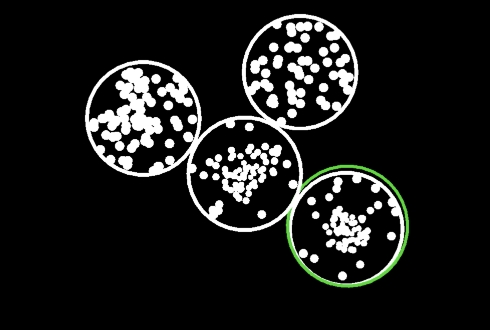
The Cognivue Clarity device takes the patient through a series of exercises to assess the domains of Memory, Executive Function/Attention, Discrimination, and Visuospatial, as well as two speed performance parameters.
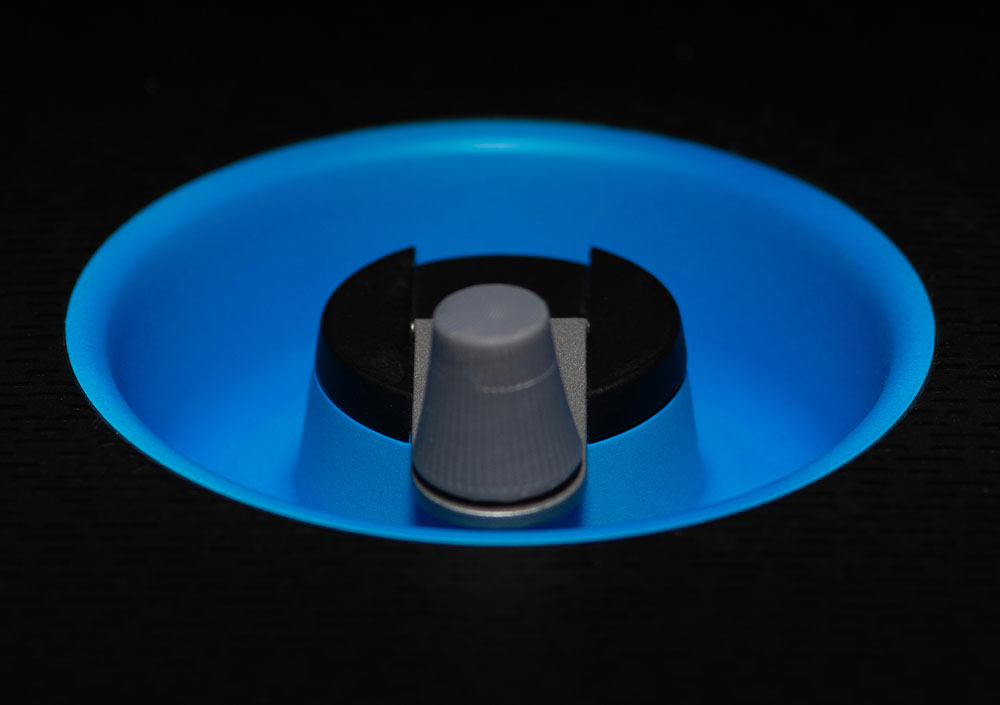
CogniWheel®
Patient interaction is through the CogniWheel, a simple, intuitive, and easy-to-maneuver wheel. This single point of contact allows patients to easily select the answer.
Cognivue Thrive® and Clarity® are indicated for use as adjunctive tools for evaluating cognitive function. They are not a stand-alone diagnostic tool and do not identify the presence or absence of clinical diagnoses. The device results are to be assessed and interpreted by a licensed clinician. Cognivue, Cognivue Thrive, Cognivue Clarity and Cogniwell are trademarks or registered trademarks of Cognivue, in the US and/or other countries. ©2024 Cognivue. All rights reserved.